Introduction :
We can classify the materials based on the electrical behaviour that we know so far into three main categories. These are
- Conductors
- Semiconductors
- Insulators
Here in this post, we will see what is so special about the semiconductor material, and why we always depend on that for our electronics needs. We will understand all these things from the fundamental level, like atomic structure.
Note :
In this entire series of posts, I am going to denote,
- Electrical conductors as conductors
- Electrical semiconductors as semiconductors
- Electrical insulators as insulators
So don’t get confused, while I represent conductors, semiconductors and insulators.
Semiconductors are an unusual materials :
Do you know what makes the conductors so special? Their ability to conduct electricity even at room temperature with the application of a small amount of electric voltage across the conductor. So we can use these types of materials for our electricity transportation. We can easily transmit the electricity from one place to another with less loss. Why is that case? Because, the conductors have a lot of free electrons, even at room temperature, with the application of electric voltage, all these free electrons will start to move in a uniform direction, so conductors start to conduct electric current with minimum loss.
What about the insulators? this material never conducts electricity at room temperature, even with the application of a large amount of voltage. So we can use these types of materials for safety purposes, like making electrical insulation from the electricity conduction wire.
Here comes the interesting type of materials whose electrical conductivity is not like conductors, but more than insulators, that is known as semiconductors. Due to this particular kind of electrical property, we can use these semiconducting materials to build our electronic systems. In semiconductors, both free electrons and holes are responsible for electric current flow.
Semiconductor atomic model :
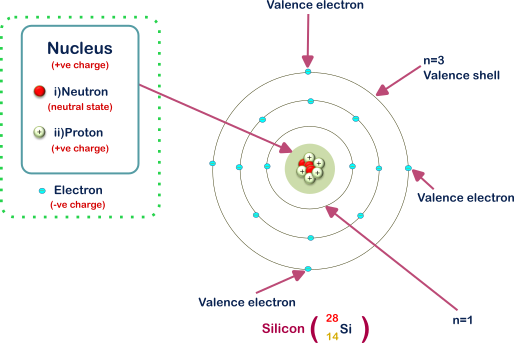
Atomic structure of semiconductors :
Generally, elements from the 3rd,4th and 5th groups of the periodic table are known to be semiconductors.
what is meant by the 3rd, 4th and 5th group of elements in the periodic table?
According to Niels Bohr’s atomic model, the electrons in the valence shell of an atom is known to be valence electron.
The number of electrons in the valence shell of an atom is,
- 3 = element is in 3rd group of the periodic table
- 4 = element is in 4th group of the periodic table
- 5 = element is in 5th group of the periodic table etc.,
So, with the number of valence electrons, the elements are grouped in the periodic table.
Some of the most used semiconductor materials
| 3rd group elements | 4th group elements | 5th group elements |
|---|---|---|
| Number of valence electrons = 3 (Positively charged due to covalent bond) | Number of valence electrons = 4 (Neutrally charged due to covalent bond) | Number of valence electrons = 5 (Negatively charged due to covalent bond) |
| Boron (B) | Carbon (C) | Nitrogen (N) |
| Aluminium (Al) | Silicon (Si) | Phosphorus (P) |
| Gallium (Ga) | Germanium (Ge) | Arsenic (As) |
| Indium (In) | Antimony (Sb) |
Here we will talk about one of the special elements from the 4th group of the periodic table, i.e. Silicon (Si).
Atomic structure of a single Si atom :
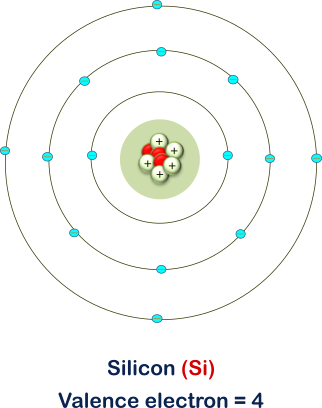
The atomic number of Si = 14, which means Si has 14 electrons and 14 protons. Therefore, there are 4 valence electrons in the valence shell of the Si atom. This is why Si is placed in the 4th group of the periodic table.
Silicon (Si) crystal view :
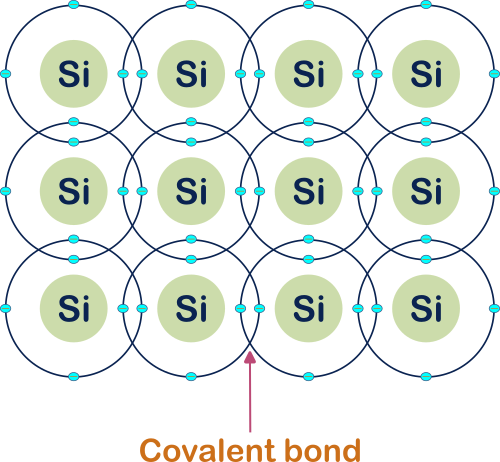
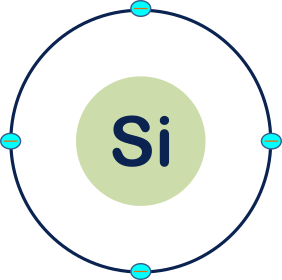
Here I am only denoting the valence shell of the Si atom.
i.e.
In the above pictures, we have seen the single Si atom’s atomic structure and how it will look when a lot of silicon atoms are placed together in the Si material.
Here an important property of semiconductors in general the Si atom in specific is shown. Every Si atom is connected with its nearby Si atom by the covalent bond. This covalent bond is the reason for the electrical conductivity of the Semiconductors in general, Si in specific.
Why do conductors have a lot of free electrons at room temperature than semiconductors?
In conductors, the neighbouring atoms aren’t in a covalent bond with each other. So, even at room temperature, the electrons in the valence shell have gained the required thermal energy to break their bond with its nucleus, to free themselves as a free electron. These free electrons are able to freely roam around inside the material. If we apply the electric voltage across the material, then we can convert these free electrons random movement into a net uniform movement to produce the electric current. But, here in the conductors, we can’t control the amount of free electrons available inside the material.
In the case of semiconductors, every neighbouring atom is connected through the covalent bond. This covalent bond makes it difficult for the electrons in the valence shell to break their bonds with their nucleus at room temperature. Even though the valence electrons in the semiconductor are getting some thermal energy from the room temperature, it is not enough for them to break their bonds. This is due to their covalent bond between the neighbouring valence electrons.
This is the reason for semiconductors not have enough free electrons at room temperature, so they can’t conduct electric current like the conductors at room temperature.
Why semiconductors are the important materials in electronics?
By now we all know that the semiconductors don’t have a lot of free electrons like the conductors at room temperature. But why it is still a perfect material for electronics in general?
We all know that free electrons in the material are responsible for the electric current flow through that material. Conductors have a lot of free electrons at room temperature, so it is best suitable for electricity conduction. But in conductors, we can’t control the amount of free electrons. In contrast, even though the semiconductors don’t have enough free electrons at room temperature in general, we can increase the free electrons amount at room temperature via a chemical operation, optical operation (or) electrical operation.
This special type of free electron generation mechanism provides us the ability to control the amount of free electrons available in the material at room temperature. Due to this, we can increase (or) decrease the amount of free electrons inside the semiconductor depends on our application.
In conductors, electrical conductivity depends upon free electrons, but incase of semiconductors the electrical conductivity depends upon both free electrons and free holes.
How free electrons are generated in the semiconductors :
In semiconductors free electrons are generated in many different ways, Those are
- Doping
- Thermal generation
- Optical generation
Difference between the conductivity levels of materials :
| Conductor | Semiconductor | Insulator | |
|---|---|---|---|
| Electrical conductivity | It is in the order of (above 103 mho/m or Ω-1m-1) | It is in the order of (103 – 10-7 mho/m or Ω-1m-1) | It is in the order of (below 10-7 mho/m or Ω-1m-1) |
| Electrical Conduction | Due to free electron | Due to both free electron and free holes | No conduction |
| Examples | Silver (Ag), Iron (Fe), etc., | Silicon (Si), Germanium (Ge) etc., | Rubber, Glass, Mica etc., |
Summary :
- Semiconductors are a special type of material, whose electrical conductivity is lesser than the conductors and higher than the insulators.
- In semiconductors, a covalent bond connects the valence electrons with the nearby atom’s valence electrons. This covalent bond makes the semiconductor a special type of material.
- In semiconductor, both free electrons and the free holes are responsible for the electrical conductivity.

